The effect of on the lower flammability limit of in atmosphere at high temperature and pressure
Hao Wei, Xianzhong Hu, Bingrong Huang
September, 2021
Abstract
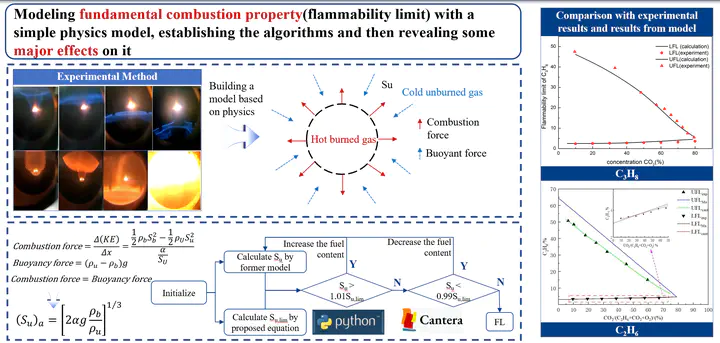
In order to provide a reference range for the safe application of under atmosphere, the lower flammability limit of was studied by calculations. A prediction model based on the limited laminar flame speed principle was used to calculate the lower flammability limit of mixture. The effects of elevated temperature and pressure on the lower flammability limit of were investigated. How the high concentration changes the lower flammability limit was also discussed. Results show that the lower flammability limit of decreased linearly with the increase of preheating temperatures. The lower flammability limit increased in a logarithmic relation with the increase of pressure. The lower flammability limit increased slightly with increasing concentration.

Computation, Modelling, and Reconstruction
 Abstract
Abstract